Register For LuxCreo's
4D Aligner™ Ambassador Program
Clinicians Driving Clear Aligner Therapy Forward
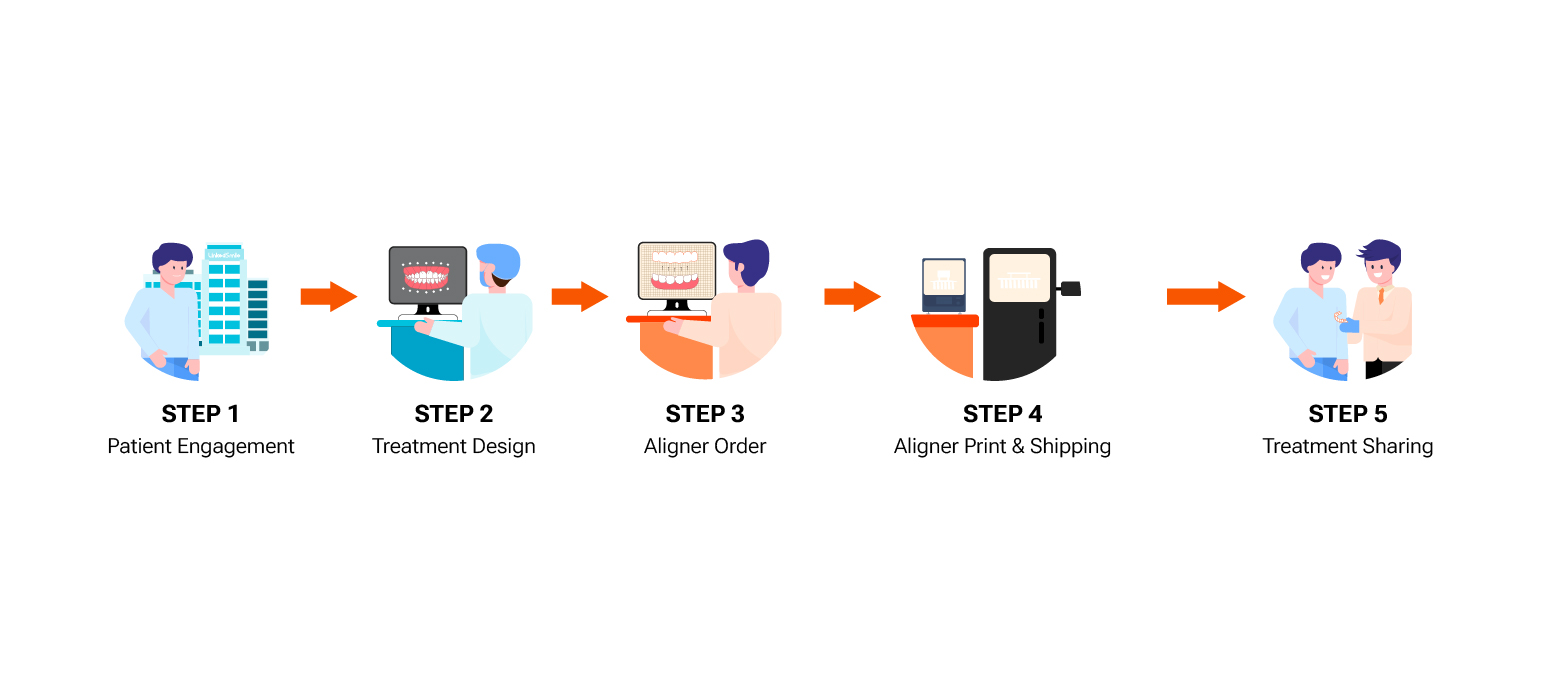
4D Aligner™ Service
What's Included Full-Service Includes
- 4D Aligner™ Treatment Planning
- 4D Aligner™ Production
- 2 Design Changes, 1 Refinement

Join the 4D Aligner™ Ambassador Program
Help Transform Clear Aligner Therapy!
User Agreement
This agreement (the "Agreement") is entered into between LuxCreo Inc. (the "Company") and the Participant, who creates a user account herein, for the purpose of providing the 4D AlignerTM product and services (the "Product") to the Participant in exchange for treatment feedback.
- Provision of Product
1.1 The Company agrees to provide the Participant with Product, including design, printing, shipping, and after-sales support, free of charge, for cases with a treatment plan within 20 steps. The Company's obligation is limited to producing the aligners based on the treatment plan provided by the Participant. The Company is not responsible for creating the treatment plan itself, which remains the sole responsibility of the Participant.
1.2 The Company shall comply with the Health Insurance Portability and Accountability Act (HIPAA) with regard to any patient information shared with the Company by the Participant. The Participant shall ensure that appropriate patient consent is obtained for the sharing of treatment information.
1.3 The Company shall promptly report any adverse events related to the use of the Product to the U.S. Food and Drug Administration (FDA) as required by applicable regulations.
1.4 The Company promises to properly use, store, and protect any patient information shared by the Participant. The Company will take appropriate measures to ensure the confidentiality and security of patient information. Any patient information shared with the Company will be de-identified and used solely for the purpose of improving the Product, research, education, or publication in professional journals. The Company will not disclose or use the patient information for any other purposes without the explicit consent of the patient and the Participant.
- Treatment Feedback
2.1 In consideration for receiving the Product free of charge, the Participant agrees to provide treatment feedback on the 4D AlignerTM, including but not limited to, its usability, effectiveness, and any suggestions for improvement. This treatment feedback may also include information regarding the patient's health and treatment results. The treatment feedback may also include patients’ orthodontic records, including photographs, made in the process of examinations, treatment, and retention. The feedback will be used by the Company for the purpose of evaluating and improving the 4D AlignerTMproduct, research, education, or publication in professional journals. The Participant understands that the treatment feedback may be used in an aggregated and de-identified manner to ensure patient privacy and confidentiality.
2.3 The Participant agrees to provide timely and honest feedback to the Company throughout the duration of their use of the Product.
2.4 The Participant shall exercise their best judgment to screen and enroll patients who are deemed suitable candidates for the Product. The Participant shall ensure that necessary patient consent is obtained in order to share treatment feedback with the Company for the purpose of this Agreement and to utilize the shared treatment feedback as defined in this Agreement.
2.5 The Participant shall maintain strict confidentiality of all treatment information and shall not disclose it to any third party without obtaining prior written consent from the Company.
- Confidentiality
The Participant acknowledges that all information and materials related to the Product, including any feedback provided, are confidential and proprietary to the Company. The Participant agrees to keep such information confidential and not disclose it to any third party without the prior written consent of the Company.
- Ownership of Feedback
The Participant acknowledges and agrees that any feedback or suggestions provided to the Company regarding the Product, excluding any patient health information or privacy-related content which remains the property of Patients, shall become the sole and exclusive property of the Company. The Participant hereby assigns all rights, title, and interest in such feedback to the Company, and the Company shall have the right to use, modify, and commercialize the feedback without any further compensation to the Participant. If the Participant wishes to use the feedback for any purpose, including but not limited to presentations, papers, or trade shows, the Participant agrees to obtain prior written consent from the Company, which may be granted or denied at the Company's discretion.
- Compliance with Laws and Regulations
The Participant agrees to comply with all applicable laws and regulations, including but not limited to the Health Insurance Portability and Accountability Act (HIPAA), in relation to the use of the Product and the provision of any feedback.
- Disclaimer of Warranty
6.1 The Participant acknowledges that the Company does not guarantee any specific treatment results or outcomes from the use of the Product. The Participant understands and agrees that the Product is provided "as is" and without any warranties or representations of any kind, whether express or implied.
6.2 The Participant expressly acknowledges and agrees that the Agreement specifically encompasses a total of 20 treatment steps, inclusive of one refinement. In the event that the patient's treatment exceeds the designated 20 treatment steps, the Company shall have no obligation to provide any further treatment without charge. However, the Company retains the discretion to offer additional treatment beyond the specified limit, subject to a separate agreement and applicable fees.
6.3 The Participant expressly acknowledges that the Product may only suitable for cases such as mild crowding, spacing, or minor tooth rotations. Severe malocclusions or significant bite issues are outside the scope of the Product under this Agreement and are not the responsibility of the Company. Such cases may require additional aligners or alternative treatments.
- Term and Termination
This Agreement shall commence on the date of signing and shall remain in effect until terminated by either party with a written notice of 30 days. The Company reserves the right to terminate this Agreement and the Participant's participation immediately without any prior notification under the following circumstances:
- If the Participant fails to provide the agreed-upon Feedback within the specified timeframe.
- If, upon evaluation solely by the Company, it is determined that either the Participant's treatment plan is not suitable for the Product or the Participant's cooperation is not suitable for the participation.
- If the Company determines, at its sole discretion, that the Participant's actions are detrimental to the Company's interests or reputation.
- If the Participant breaches any terms or obligations outlined in this Agreement.
Upon termination by the Company, the Participant shall return any remaining Product and cease using the Product.
- Governing Law
This Agreement shall be governed by and construed in accordance with the laws of California, without regard to its conflict of laws principles.
- Entire Agreement
This Agreement constitutes the entire understanding between the Company and the Participant with respect to the provision of the Product in exchange for user feedback and supersedes all prior agreements, understandings, or representations, whether oral or written.
Your creation of a user account indicates your acceptance of the terms and conditions of this Agreement. Upon request, a full copy of the Agreement will be sent to you.
Thank you for your participation in providing user feedback for the 4D AlignerTM. We look forward to a successful collaboration.